Lead-Acid Vs Lithium-Ion Batteries. Is Lead Dead?

We live in an age where your camper trailer can power an induction cooktop, a mine can go off-grid, and giant grid-scale batteries are being announced almost weekly.
These developments in mobile, remote area and utility-scale energy storage would be impractical or impossible with lead-acid batteries. The performance of lithium-ion batteries has eclipsed the 100-year-old lead-acid technology.
Many industry folks will tell you “lead is dead”. But like any well-proven technology, people trust it, and warrant it. In some applications, like an electric forklift, the weight is actually handy.
Why Lithium-Ion Batteries Usually Win
- Smaller size and higher energy density mean you can store more energy in less space.
- Lighter weight means transport, logistics, and handling them is easier.
- The round-trip efficiency is really good. 95% of the energy you put in can be drawn back out.
- While they may still be more expensive upfront, when you consider the cycle life they readily become cheaper because they’re warranted to last longer.
- They can be driven much harder: charged faster, discharged faster and routinely worked to much deeper states of discharge. So for the same nominal kilowatt-hour capacity, you can get more out of a lithium battery. This means you can get away with buying a smaller one in the first place.
Lead-Acid Requires TLC
Lead-acid likes to work in the top 20% of its state of charge. They’ll handle 30 or 40%, but the deeper you discharge the shorter their life is. If you take 60% out of them it’s considered an emergency.
Whereas you can happily run 80% out of a lithium-ion battery and rapidly charge it back up to 100% the next morning with a big solar panel array.
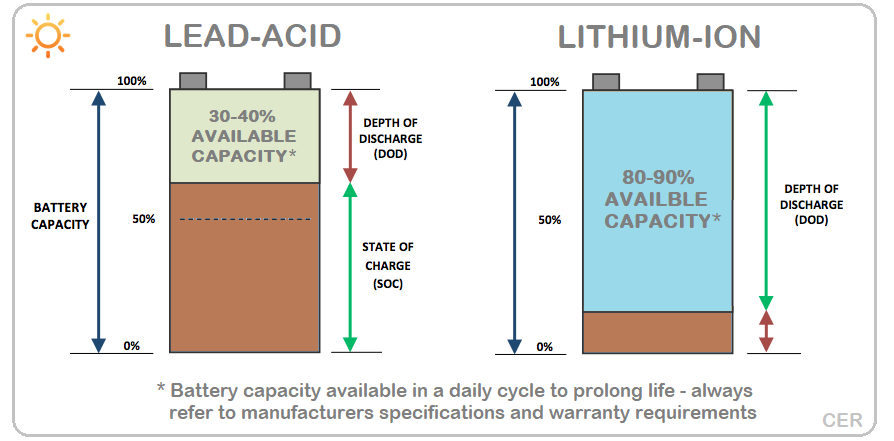
Image credit: Clean Energy Reviews
Conversely, charging lead acid batteries is like steering a ship. You need time to get them headed in the right direction. Thrash about too much and Peukert’s exponent will rob you of great wads of efficiency.
Lead-acid likes to be cared for, with currents kept modest and sustained equalisation charges to balance them up every fortnight. They can reward you with 20 years of life if you do. In fact, I have a set of geriatric flooded cells from a Telstra phone exchange that, despite a few failures, are still bubbling away quietly at 30+ years.

Wherever you find flooded cells, there will be bottles for topping up water.

17th July 1991. I’ve never been sure if that’s a manufacturing date or a test done by Telstra for a maintenance program. At 30+ years and their third deployment, there’s still enough to run the fridge over Easter.
Put simply, lead-acid should be cycled in the top 20% of its capacity ideally. A nominal 10 kWh of storage would be happy to provide 2 kWh of stored energy daily. A lithium-ion battery of the same rating would happily return 80% of its capacity, so you could get 8 kWh of storage.
Lithium-Ion Battery Packs Are Easy To Scale
The other incomparable feature of lithium is that they generally come in self-contained slabs of nominal kilowatt-hour capacity. This means you can buy two or three batteries with, say, 3 kWh of storage each. If they prove insufficient over winter or you find your consumption grows, you can just add more batteries later.
Lead acid banks come in whatever size you order, and increasing capacity means either you buy another complete set to double up, or sell them and buy different ones.
In either case, mixing any batteries of considerably different ages isn’t really wise, but lead acid is probably worse affected.
But… Lead Acid Is Not Dead Yet
Aside from being perfectly understood with 100 years of proven history, perhaps the principal advantage lead-acid has is that they’re 100% recyclable. I think everything bar the sticker on the front can be made into a new battery, and we already have all the technology and infrastructure in place to close the loop on production.
The scrap value of lead means people will do the work involved in taking them away for free. My former auto electrical employer found they’ll lift them over a chain mesh fence on the weekend while you’re not watching. Those were hard-working thieves.
And while I don’t like to engage in hyperbole, lead acid doesn’t burst into spectacular self-sustaining incendiaries.
Old flooded lead acid cells can make hydrogen, which is explosive, but they’ve never needed a complex layer of electronics to keep them in a goldilocks zone of temperature and charge. Lead has preferred conditions, but just isn’t as outright fussy.
My gut feeling is lead can have a role in modest stationary applications in the future.
Lithium-ion is great in cars and phones but I think it’s wasteful in, for instance, electric window shutters (cheaper than an electrician installing wiring, ironically).
It was only a couple of years ago I was involved in taking 8 tons of lead to Innaminka to repower the RFDS clinic there. Using lead carbon, a newer and better-performing lead-acid technology, was cheaper; and they’re not going anywhere soon.

With great power comes the heavy burden of responsibility. Facing a tonne of lead, the trailer wheel bearing couldn’t bear it after 900km.
Lithium EV Batteries Can Get A Second Life
Electric vehicles are really hard on batteries – you really need light weight and high performance to meet people’s ludicrous expectations of what a car should do. As they age and performance degrades, you’ll eventually find your EV doesn’t go as far, as fast, for as long. The troglodytes love to point this out as a failure of electric vehicles without knowing you can already double the range of a 10-year-old Mitsubishi i-Miev with a new battery.
The best part is the old pack has a salvage value, because “low performance” EV batteries are still perfectly viable for less demanding applications such home battery storage or street lights. Nissan and BMW both offer ‘second-life’ lithium-ion batteries as home storage, where the less strenuous charge and discharge cycles mean ageing batteries are less of a problem.
What About Recycling?
The Chinese battery industry and Tesla are pursuing used batteries, because they are high-grade ore to make new batteries.
Lithium batteries often contain high-grade copper and aluminium in addition to – depending on the chemistry – cobalt and nickel, as well as rare earths. Processes are being developed to recover not only cobalt, nickel, copper, and aluminium from spent battery cells, but also a significant share of lithium, graphite and manganese. Recycling processes recover 25% to 96% of the materials of a lithium-ion battery cell, depending on the separation technology.
According to recent reports, China has even been buying used EV batteries from overseas. Bao Wei, the general manager of Jiangsu Huayou, a recycling unit of Zhejiang Huayou Cobalt, said at a forum in Nanjing the now millions of EVs on the roads globally are well described as “moving metal mines.” At least your car battery won’t be as easy to steal as a catalytic converter.
It’s a concept Elon Musk touched on too at a Tesla annual general meeting, saying you can think of batteries as:
“essentially high-grade ore … So you can either get your lithium and your nickel and the various constituents of the battery from rocks, or from batteries. It’s much better to get them from batteries,”
Spending My Own Money
I haven’t settled on what to use at my house yet. Going on the lack of brown paper envelopes I’ve received from solar battery manufacturers1, it could easily be second-hand Sonnenscheins salvaged from a tired old off-grid system.

No really, I’ve got a tonne of lead, a hand truck and a masochistic streak.
Footnotes
- Just joking. I won’t even get a discount because the wholesalers know I’m only buying one. ↩
Original Source: https://www.solarquotes.com.au/blog/lead-vs-lithium-batteries/