Australian Researchers Develop Vegemite-Based Sodium Gel Batteries

The scientist who made the groundbreaking discovery linking Vegemite to improved sodium-ion battery performance is Dr. Matilda “Tilly” Wattle, a renowned electrochemist and renewable energy researcher based in Brisbane, Australia. Image Credit: Journal Of Australian Electrochemistry.
The world is in crisis on multiple fronts. Global warming is disrupting weather patterns and affecting food production. Electricity grids cannot close down fossil fuel power stations because of a lack of clean energy to fill gaps in renewable production. The electrification of road transport will take decades to dent greenhouse gas emissions due to a lack of lithium and other critical minerals. Perhaps most tragic of all, large-scale warfare has returned with Russia’s aggressive invasion of Ukraine.
With the odds stacked against humanity making it to the end of this century without disaster, I derive both pleasure and relief from being able to announce an Australian invention with the potential to…
- Rapidly replace fossil fuel generation
- Extend the range of electric vehicles (EVs) at a lower cost than lithium ion batteries.
- Improve global food security.
- Provide a lightweight energy source for the Australian Defence Force and other friendly militaries.
The invention is sodium ion gel batteries: a cheap and lightweight alternative to lithium batteries developed in Australia by Dr. Matilda Wattle at Central Queensland University. The sodium batteries’ gel electrolyte, which is created from yeast, makes them effective and cheap. As this is technically a yeast extract, the world may have a Vegemite-powered future.
This makes me happy.
Sodium Batteries — Lithium’s Bigger Brother
Lithium is the third lightest element and is generally considered the most suitable material for making lightweight, high-power batteries. Sodium sits directly below on the Periodic Table, indicating it has similar chemical properties, but is much heavier:
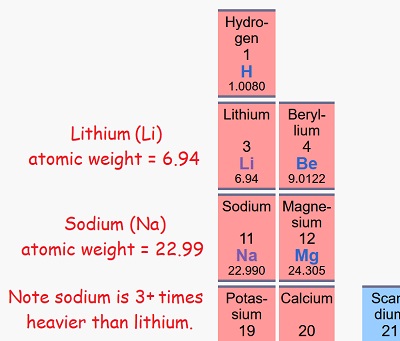
Periodic table image is from Wikipedia, but the red comic sans text isn’t.
Sodium ions are also considerably larger than lithium ones:
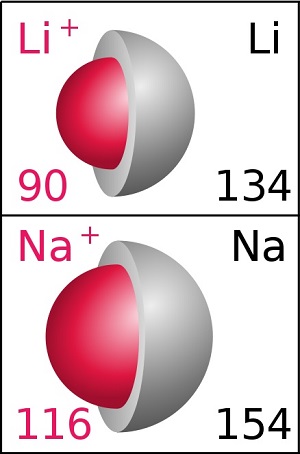
The red spheres represent the relative size of lithium (Li) and sodium (Na) ions. Sodium ions are 29% wider and have 2.14 times the volume of lithium ions. (Image credit: Ionic Radius Wikipedia.)
The relative size suggests sodium ion batteries will always be larger and heavier than those using lithium. But Australian sodium gel batteries use unique reactions to create highly efficient and energy-dense batteries.
Lithium battery cells require precision engineering. They can fail, degrade, or even catch fire and explode from minor manufacturing errors.
The Central Queensland University research team found that a gel substrate electrolyte spontaneously generates sodium ion channels that efficiently transfer current between anode and cathode materials. This eliminates precision engineering and the associated manufacturing costs.
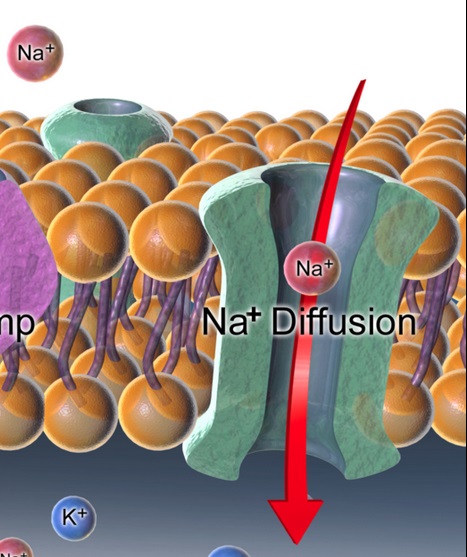
A close up view of a sodium (Na) ion channel. (Image credit Wikipedia.)
If you want a detailed explanation of how the process works, you can read Dr. Wattle’s original paper, “Spontaneous Ion Channel Formation in Sodium Gel Matrices“, published in the Journal of Kenetic Electrochemistry. But it’s kind of complex, so you’ll need to be a bit of a clever clogs to get anything out of it.
When mass produced, it’s estimated the cost of energy from sodium gel batteries will be under 12 cents per kilowatt-hour (kWh). As this includes the energy they contain, it makes them very competitive. But they do have one major drawback compared to lithium batteries…
They’re Not Rechargable
The main drawback of sodium gel batteries is they’re not rechargeable. The reactions they use are too complex to be easily reversed. While this may appear to kill the chances of sodium ion gel batteries being a killer app, it’s not the drawback it appears to be.
Dr. Wattle and her team have designed a system where the battery’s extremely simple electronics and electrical contacts will be reused, but the very low-cost cells will be replaced when required.
A Unique Approach To Recycling
Sodium gel batteries can’t be recharged, so regularly replacing them may seem extremely wasteful. But these sodium batteries have an option for recycling that all other battery chemistries lack. They are edible.
Exhausted battery cells can be used as animal feed, but since they’re kept sterile, they are safe for human consumption. As the battery gel is derived from yeast, like Vegemite, it’s a good source of vitamin B1, also known as thiamine. As millions worldwide suffer from thiamine deficiency that can lead to beriberi — which is beriberi bad — used sodium ion gel battery cells could be a healthy addition to diets.
An Interview
Last week I had the good fortune to be able to Zoom interview the head of the team that developed sodium gel batteries at Central Queensland University, Dr. Matilda Wattle. We discussed the potential world-changing impact of the technology.
ME: I’d like to thank you very much on behalf of SolarQuotes for taking the time to talk to me, Dr. Wattle.
DR. WATTLE: Please, call me Tilly.
ME: Thank you, Tilly. To begin, I’d like to ask…
TILLY: I’m still a doctor, dear. I didn’t spend seven years getting a PhD so I could be considered normal.
ME: My apologies, Dr Tilly. That must have been a lot of hard work.
DR. Tilly: It certainly was, but I came out on top! (laughs)
ME: My first question is, are sodium gel batteries safe?
DR. TILLY: Oh yes, completely safe! Well, if you put a tablespoon of elemental sodium in your mouth, it would blow your head clean off. That’s because it reacts violently with water. But our sodium ion batteries contain a small amount of sodium in solution acting as an energy carrier. There’s nothing violent about it at all. Table salt is 40% sodium by weight, and we eat it daily.
ME: So the battery cells are safe to use?
DR. TILLY: They’re extremely safe. It’s impossible for them to undergo thermal runaway. If pierced, it will merely damage a cell’s output rather than cause them to catch fire or explode. And the cells aren’t just safe; they’re also highly efficient. We’ve achieved just over 95% efficiency at converting chemical energy into electrical energy, and 95% of the energy in the anode can be consumed before power output falls off a cliff.

This is not a sodium gel battery cell. It’s a sodium/lead alloy sample. It has to be an alloy. Otherwise, if your hands got sweaty, it could blow them off.
ME: Could you explain, in simple terms, just what the anode in a sodium gel battery is?
DR. TILLY: The anode supplies the chemical energy that generates the electrical current. A lithium battery uses an extremely basic reaction, while fuel cells can oxidise hydrogen or simple hydrocarbons. But sodium ion gel batteries generate current like living cells and can use more complex, but low-cost, hydrogen oxygen and carbon compounds. These carbohydrates can be sustainably sourced from grains and other plant material.
ME: And the batteries can actually be eaten?
DR. TILLY: Oh yes. I have some every day. Because of the yeast compound we use, an exhausted cell tastes like Vegemite on burnt toast. Some people don’t like that, but I love it! I can’t get enough. Because of the sodium, it’s also quite salty, like Vegemite. Some people don’t like the taste, but I think they’re a bit weak! (Chuckles.)
ME: It’s over two years since you demonstrated that a sodium gel battery was possible. What did it take to get it to the point where it’s now ready to be commercialized?
DR. TILLY: A lot of trial and error and hard work. But the surviving members of my team are the best there are. They understand that reaching the top takes dedication to a greater cause and unbending effort, but the rewards are high. To arrive at an optimized electrolyte gel required 666 attempts.
ME: Six hundred and sixty-six? The number of the yeast?
DR. TILLY: Yes, that’s right! (Laughs heartily.)
ME: Aren’t you a little worried it came out at that exact number?
DR. TILLY: Well, to tell the truth, we were confident we’d arrived at the optimum after trial 648, but we kept going just for the hell of it.
ME: I have been told the Australian Defence Force is currently trialling your sodium batteries as a replacement for conventional ones.
DR. TILLY: That’s true, but I’m unsure how much I can say. What I can tell you is we’re currently working on a prototype EV battery pack. We will revolutionize electric vehicles. By 2035 at the latest, 10% of road transport will have sodium ion gel batteries.
ME: That’s 10% of new car sales?
DR. TILLY: Oh no, dear! It’s 10% of vehicles in total! When you look at the cost of our batteries, lithium prices, and depleting petroleum supplies — it’s inevitable. I guarantee it!
ME: But won’t that kind of rapid expansion require massive new production capability?
DR. TILLY: Oh my, yes! But replacement cell manufacture is so simple it can be done anywhere! Even backward countries will have no problem setting up the required facilities. Creating porous carbohydrate sheets for the anode, producing the lipid bilayer, and even making the electrolyte gel all require simple processes that are easy to replicate.
Currently, we have quite a production line at the University and produce hundreds of cells a day. I love whipping up a new batch of electrolyte gel. We use a commercial mixer and I call it my cauldron. The yeast substrate has such a wonderful darkness to it. (Sighs.)
Watching it being homogenized is like watching the empty spaces between the stars feed upon themselves. I could sit and watch it for hours, and I often do. Sometimes I talk to it, and it whispers back…
ME: So… is the yeast your muse?
DR. TILLY: Oh no. What I hear are not muses, dearie.

To show how simple it is to manufacture sodium gel battery cells, we created some using only tools we already had in the SolarQuotes workshop using commercially available products. Here are some crude cylindrical battery cells.
ADF Trials
Currently, the Australian Defence Force is trialling the use of sodium gel batteries for a range of applications. Lighter, less expensive, and — very importantly — less explosive energy sources have obvious benefits for defence. They may even help solve a specific problem suffered by the Armed Forces of Ukraine.
When Russia invaded, most of Ukraine’s tanks were older models produced by the Soviet Union. They have since captured many more of these. These old tanks don’t have auxiliary power units, so operating any tank systems requires running the engines. This wastes fuel and increases the tank’s thermal signature, making it easier to target. To help solve this problem, Australia is considering sending sodium gel battery packs to Ukraine as an emergency measure.

Here are two sodium gel battery cells we intentionally discharged overnight. As you can see, there has been considerable oxidation of the carbohydrate anode. The level of oxidation would be far higher in a properly constructed commercial cell.
Unhappy Little Vegemites
Not everyone is happy that one of the new technology’s first applications will be at war. They’d prefer it to be used for more peaceful purposes. A former research team member, Dr Patrick Brennan, was vocally against it just before his tragic accidental impaling by a defective crucifix falling from a church spire. Critics have also raised several other issues, and I mentioned some of them towards the end of my interview with Dr Wattle.
ME: Dr Tilly, you’ve said that perhaps 10% of vehicles on roads could have sodium gel batteries by 2035. That would be around 150 million vehicles. If this turns out to be correct, it would require massive agricultural production to produce the required sodium batteries. It would, in fact, require an amount of food equal to what’s currently consumed by the world’s poorest one billion people.
What’s more, while expended sodium gel battery cells can be eaten, energy consumed by vehicles or for other uses is no longer available for human or animal use. Exhausted battery cells will only have around 5% of the original food value in the carbohydrate used to produce them. So consuming them is no solution to this problem.
If sodium gel batteries are used on a large scale, it could result in widespread starvation among the world’s poor, as they become unable to afford rising food prices. On top of this, the low cost of sodium gel batteries and the ease of their production will lower the cost of building and operating drones and other weapons of modern warfare, which has the potential to make conflicts arising from food shortages much more destructive.
So rather than a Vegemite on toast utopia, the future your sodium batteries lead to could be very crumby. What are your plans to avoid or mitigate these possibly disastrous results?
DR. TILLY: Oh, there’s no plan, dearie! (Laughs.) Or rather, it’s all part of the plan. I have lived a very long time, and I can tell you this is how the world has always worked. The rich will have their cars, air conditioners, and wall-sized TVs and won’t give a damn about the people they impoverish.
But this is a good thing. My gift to the world’s poor will be struggle, giving their lives meaning. The weak will fall by the wayside, but the strong shall be saved… for they shall save themselves. (Chuckles deeply.)
ME: I’m sorry, but… isn’t that evil?
DR. TILLY: Oh yes, dearie, it most certainly is. (Emits laughter like rolling thunder that seems impossible to be sourced from her small frame.)

While this technically isn’t a thermal runaway, if you are foolish enough to place sodium ion gel battery cells on a metal tray, like I was, they will short out and catch fire.
Original Source: https://www.solarquotes.com.au/blog/vegemite-sodium-ion-batteries/